The innovation of the patented VABECK® PROCESS is the development of a new process that enables high gas temperatures to be achieved with minimal heat losses and the separation of reaction products. This process enables sustainable, climate-friendly and economical hydrogen production through thermal water splitting, methane pyrolysis or ammonia cracking.
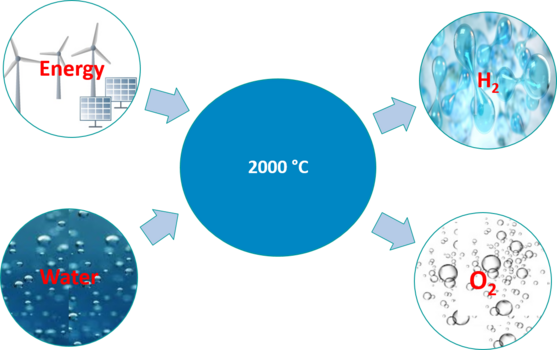
In thermal water splitting, water is thermally split into hydrogen and oxygen in the water splitting reactor at temperatures of approx. 2000 °C without catalysts, whereby the reaction products are separated and the hydrogen is exported from the reactor separately from the oxygen. No expensive heat-resistant membranes are used to separate hydrogen and oxygen.
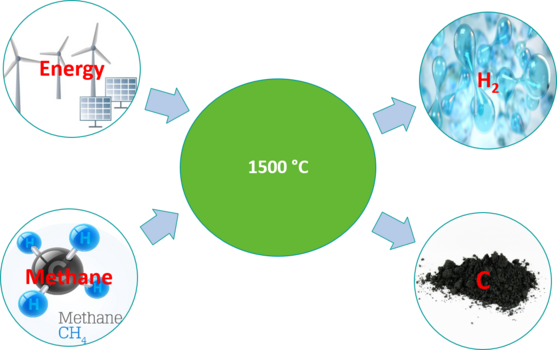
During thermal decomposition, methane is decomposed directly into hydrogen gas and solid carbon at high temperatures in a CO₂-neutral process. There is no clogging of the reaction zone with carbon, which is a major problem with other pyrolysis processes. This process produces no direct CO₂ process emissions and even negative emissions if biomethane is used as a raw material and renewable energy.
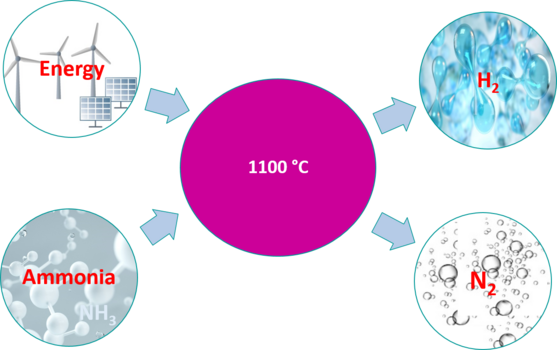
Ammonia (NH₃) can be produced from renewable hydrogen (H₂) and atmospheric nitrogen (N₂). Ammonia is easier to liquefy than H₂, boiling point under atmospheric pressure -33 °C, and is well suited for storing and transporting hydrogen. After transporting the hydrogen in the form of ammonia, it is converted back into gaseous hydrogen at low cost by VABECK® PROCESS.
But ... conventional H₂ production by steam reforming (gray) is harmful to the climate: 1t H₂ → 10t CO₂
But ... H₂ production with CO₂ capture (blue) is controversial and not yet economically established
But ... climate-friendly production with electrolysis (green) only exists in pilot plants and is currently uneconomical
But ... other production processes such as methane pyrolysis (turquoise) are still being researched
But ... there is no production of green hydrogen on an industrial scale anywhere in the world
